A New Lens on “Getting Old”
The Information Theory of Aging (ITOA) flips the usual script. Instead of blaming frayed genes, it argues that we age because the instructions for reading those genes—the epigenome—slowly scramble. Your DNA is like rock-solid hardware, but the epigenome is the software overlay of chemical tags and loops that decides which genes play and which stay silent. Unlike DNA, that software is wonderfully flexible—and therefore painfully vulnerable to stress, sunlight, diet, and time.
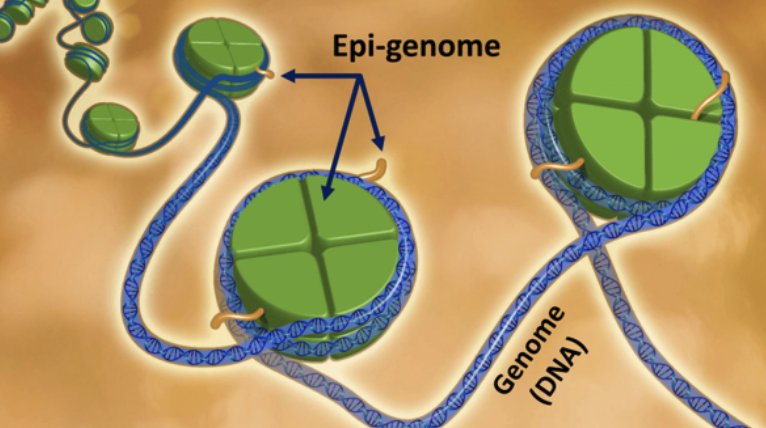
When the Software Glitches
Every day, double-strand breaks (DSBs) gouge the genome. Emergency responders such as sirtuins sprint to the damage, patch it, then try to hustle back to their posts regulating gene expression. They don’t always land exactly where they started, leaving behind tiny “typos” in the epigenetic code. Over decades those errors pile up into epigenetic noise, and cells begin losing their professional identity in a process called exdifferentiation. The result is a cascade of classic aging hallmarks, from inflammation to stem-cell exhaustion.
Claude Shannon Meets the Cell
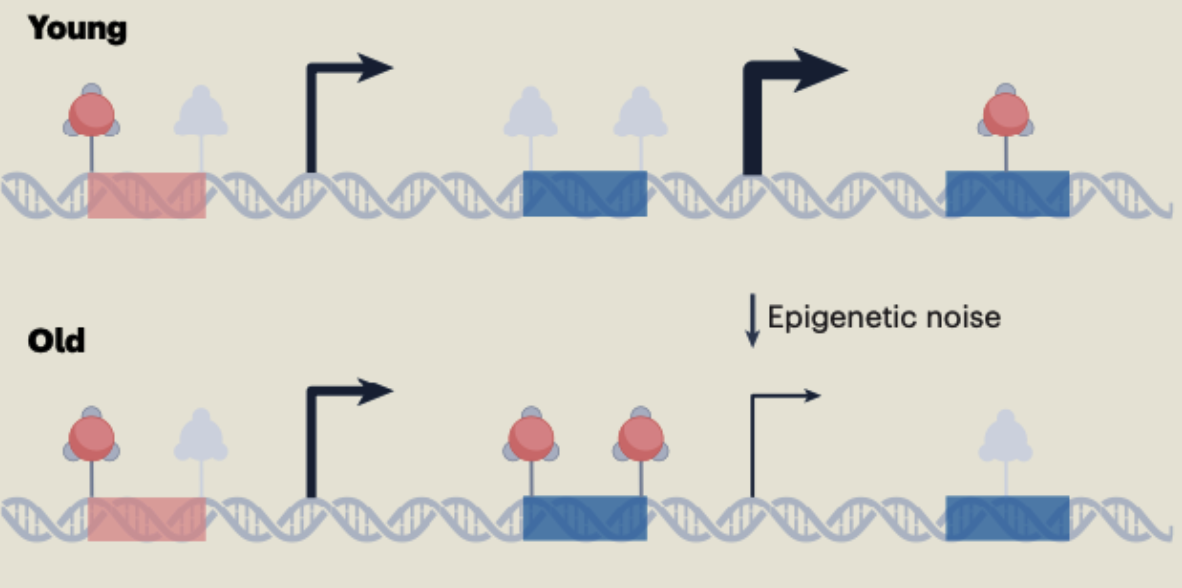
In the 1940s, Claude Shannon proved that any signal breaks down as background noise rises. The Information Theory of Aging (ITOA) repurposes that insight: our genetic “broadcast” remains intact, yet it’s muffled by epigenetic static. Picture an old CD—its songs are still etched into the disc, but scratches fill the speakers with clicks and pops. Buff the surface, and the music plays crisply again. Shannon also showed that an “observer,” or error-correcting backup, can retrieve a corrupted message. ITOA suggests biology has its own safeguard: a pristine backup copy of our youthful epigenetic information tucked inside every cell. If scientists learn to tap this backup, they could reboot healthy gene expression and revive cellular function.
Rewinding with Partial Reprogramming
The most convincing evidence? Partial epigenetic reprogramming. Brief pulses of the Yamanaka trio OSK (OCT4, SOX2, KLF4)—minus the cancer-prone MYC—have reset aged mouse eyes, reversing blindness and rolling back DNA-methylation clocks without erasing cell identity. Delivered via controllable viruses or even small-molecule cocktails, OSK appears both potent and safe.
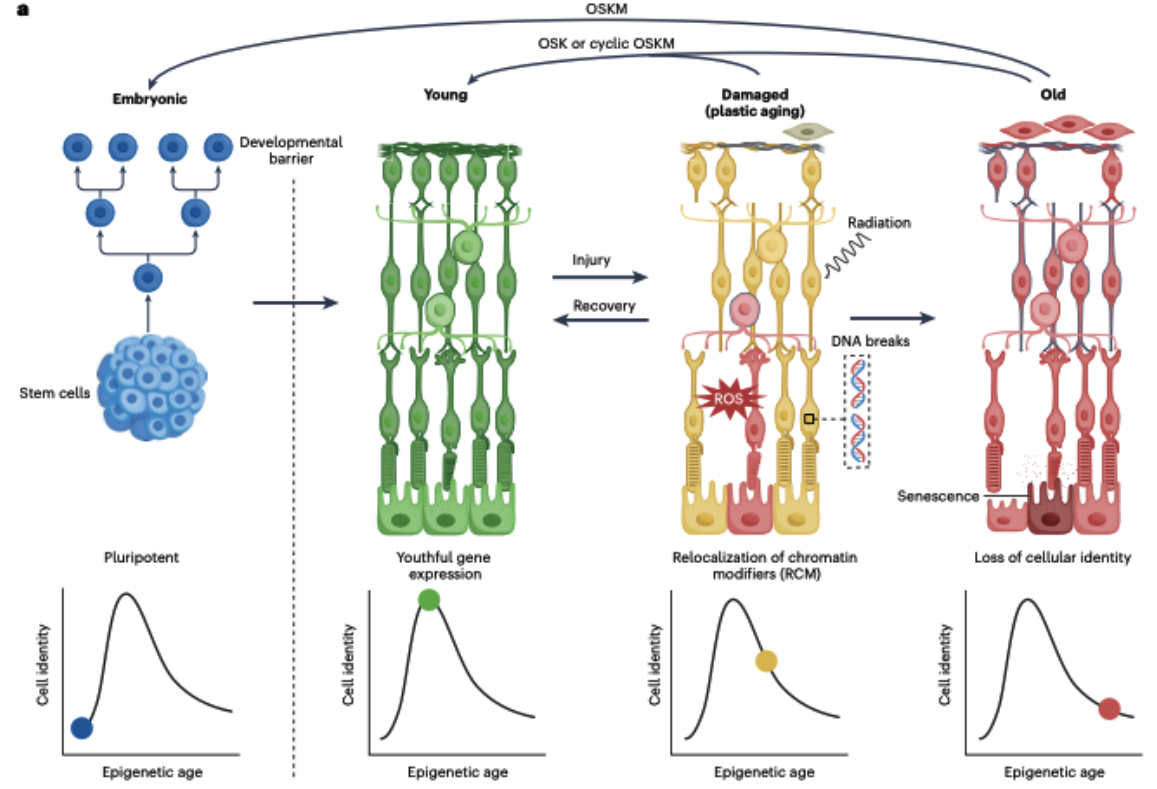
Why It Matters
Because epigenetic age is plastic, debugging it could treat degenerative diseases at their root, extend healthy years, and maybe let future seniors feel more like experienced rookies than worn-out veterans. Human trials aimed at reversing blindness are already in motion, and chemical reprogramming hints at pill-based rejuvenation down the road. For the first time, “growing old” looks less like inevitable rust and more like a solvable software bug.