Aging 2.0—A Software Bug, Not a Hardware Crash
What if aging isn’t just about your body wearing out, but about your cells forgetting how to be young? Imagine aging not as a relentless breakdown, but as corrupted software—glitchy, scrambled, out of sync. Now imagine we could restore that software. Debug it. Even rewind it. That’s the radical (and evidence-backed) idea behind the Information Theory of Aging, or ITOA. It suggests that aging is driven by the loss of “epigenetic information”—the very code that tells our cells who they are and what to do.
Hardware vs. Software: Genome ≠ Epigenome
Our genome is like the hardware: a fixed instruction manual made of DNA. But right on top of the genome sits the epigenome—a system of chemical tags, DNA loops, and proteins that controls how and when genes are switched on or off. Crucially, unlike DNA, the epigenome is plastic—and therefore vulnerable to stress. Over time, it accumulates epigenetic noise from UV light, poor diet, and sheer time.

What Is the Information Theory of Aging (ITOA)?
The ITOA says this: Aging happens not because your genes fall apart, but because your cells lose the instructions for reading those genes.
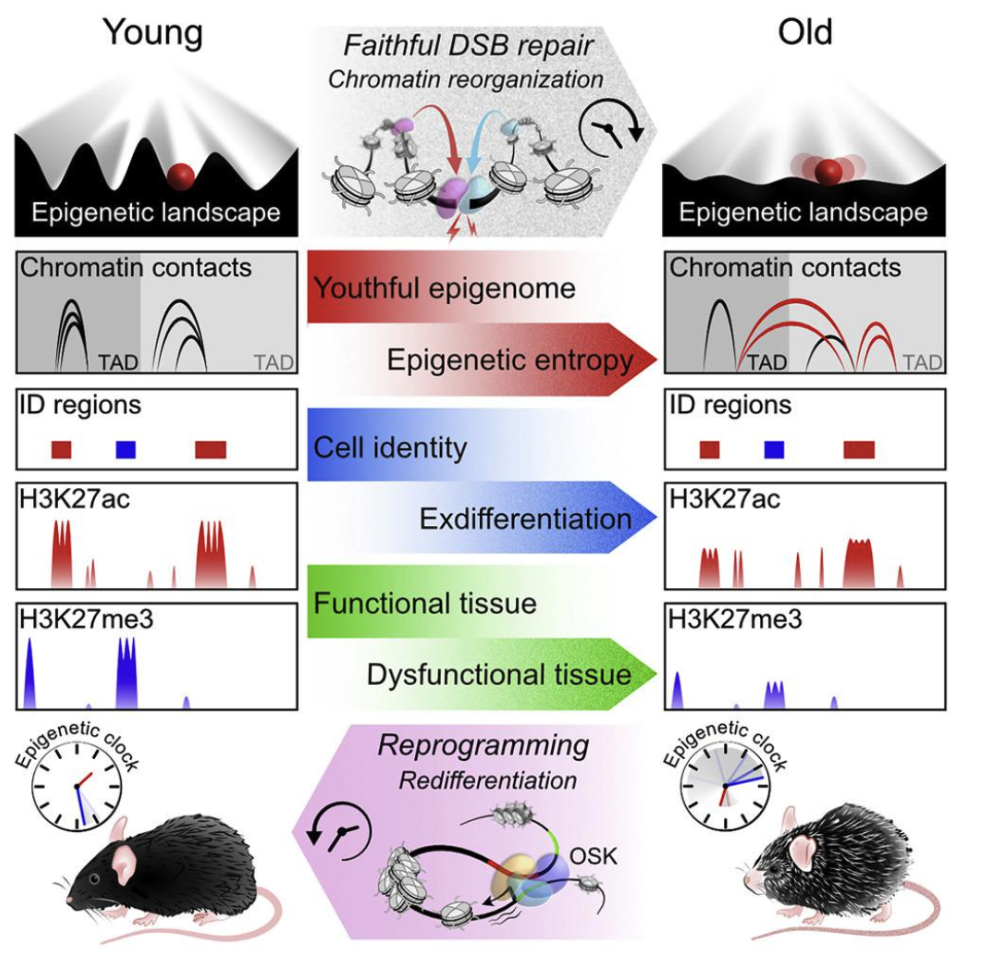
Every day, thousands of double-strand DNA breaks call repair crews away from their posts regulating gene expression. Key chromatin modifiers—including the longevity proteins called sirtuins—rush to the damage, patch the DNA, but don’t always return to exactly the same addresses. Each trip adds a speck of epigenetic noise. Over decades, the three-dimensional epigenetic landscape—known as Waddington’s hills and valleys—erodes, and cells slide into the wrong valleys, a drift dubbed “exdifferentiation.”
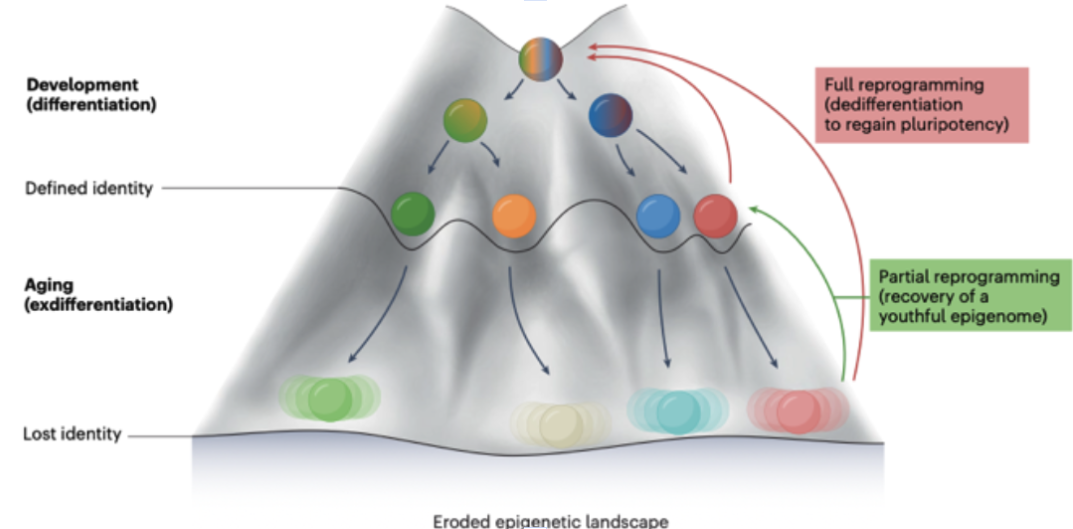
When cells lose their epigenetic instructions, they can no longer perform their specialized functions properly. And when that happens, cells begin to lose their identity, triggering many of the hallmarks of aging.
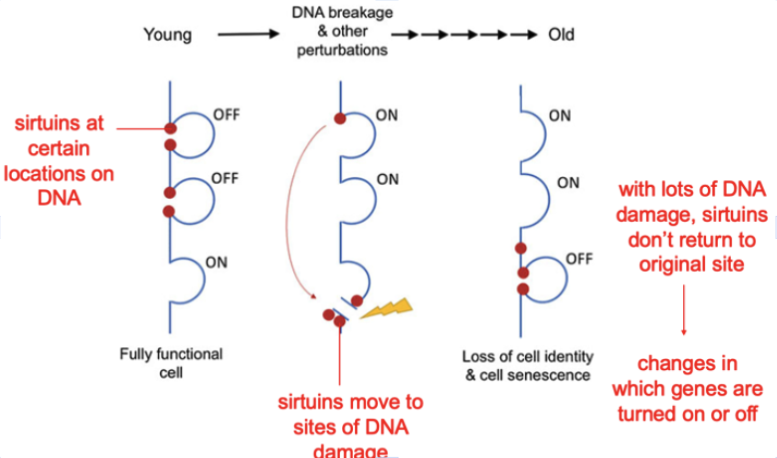
The Backup Plan
Claude Shannon showed that any message degrades as noise grows. ITOA borrows that concept: the genetic “signal” is intact but buried under static. Think of a scratched CD—the music is still encoded, yet clicks and pops ruin playback. If we polish the disc, fidelity returns. Likewise, cleaning the epigenome could let youthful gene expression play again.
Shannon’s work in the 1940s showed that if you have an “observer”—a backup copy that corrects errors in transmission—you can restore the message. ITOA asserts that something similar happens in biology: a backup of our youthful epigenetic information hides in our cells, waiting to restore function.
Can We Rewind the Epigenetic Clock?
Yes. And the key could lie in partial epigenetic reprogramming. In 2006, Shinya Yamanaka discovered that four factors (OCT4, SOX2, KLF4, and MYC) could reset an adult cell to a pluripotent state. But wiping the slate clean isn’t ideal inside a living body.
Partial epigenetic reprogramming—briefly expressing OSK (no MYC)—can reset the epigenetic clock without erasing identity. Applied correctly, OSK reverses signs of aging in mouse eyes, muscles, and beyond, rebuilding the epigenetic landscape and guiding cells back to youthful paths.
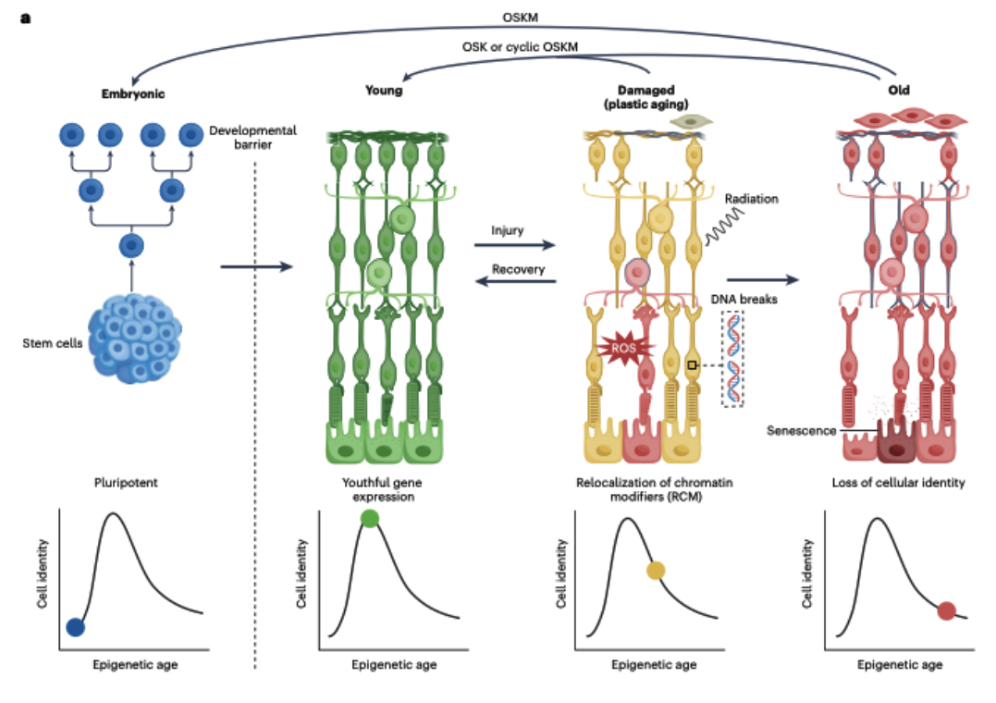
Support for the ITOA: Evidence That Youth Is Backed Up
- Nature’s Reset Button: During fertilization, the germ line gets a complete epigenetic reset.
- Cloning (Dolly the Sheep): Adult DNA can grow a new, young organism.
- Reversing Blindness (2020, Nature): OSK restored vision in aged mice.
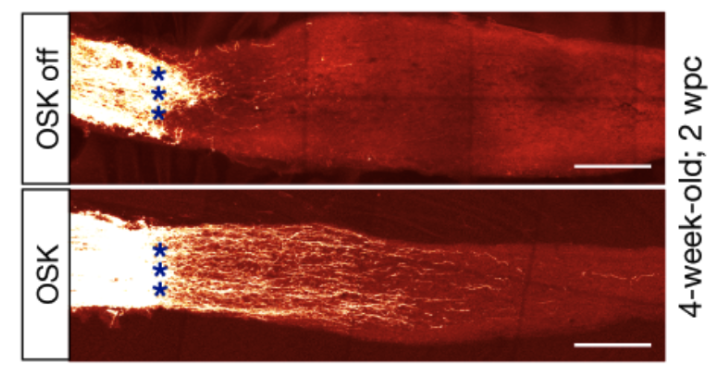
- ICE Mice: Inducible epigenetic damage sped aging; OSK reversed it.
- Chemical Reprogramming: Human fibroblasts rejuvenated in four days via small-molecule cocktails.
Why ITOA Matters
If aging is an information-loss bug, it’s debuggable. Resetting epigenetic code could treat degenerative diseases at their root, restore organs without gene editing, and enable affordable, safe rejuvenation. Human trials targeting blindness are already in motion; systemic rejuvenation may not be far behind.
Conclusion
The Information Theory of Aging reframes senescence as misplaced punctuation in life’s manuscript. By repairing the formatting—not rewriting the words—we might turn back the biological clock. Youth is still in there, waiting for a skillful editor to retrieve it.